One oncology platform, multiple purposes
A tumor xenograft that cuts therapeutic efficacy testing from 6+ months to 5 days

Zebrafish embryos are utilized to develop a rapid xenograft in 5 days. The zebrafish xenograft then can be cultured in CTX, PTX, or patient tumors for immuno-therapeutic development.

The ZTX® platform has the advantage of stable and scalable human immune cell hosting in the xenografts produced.
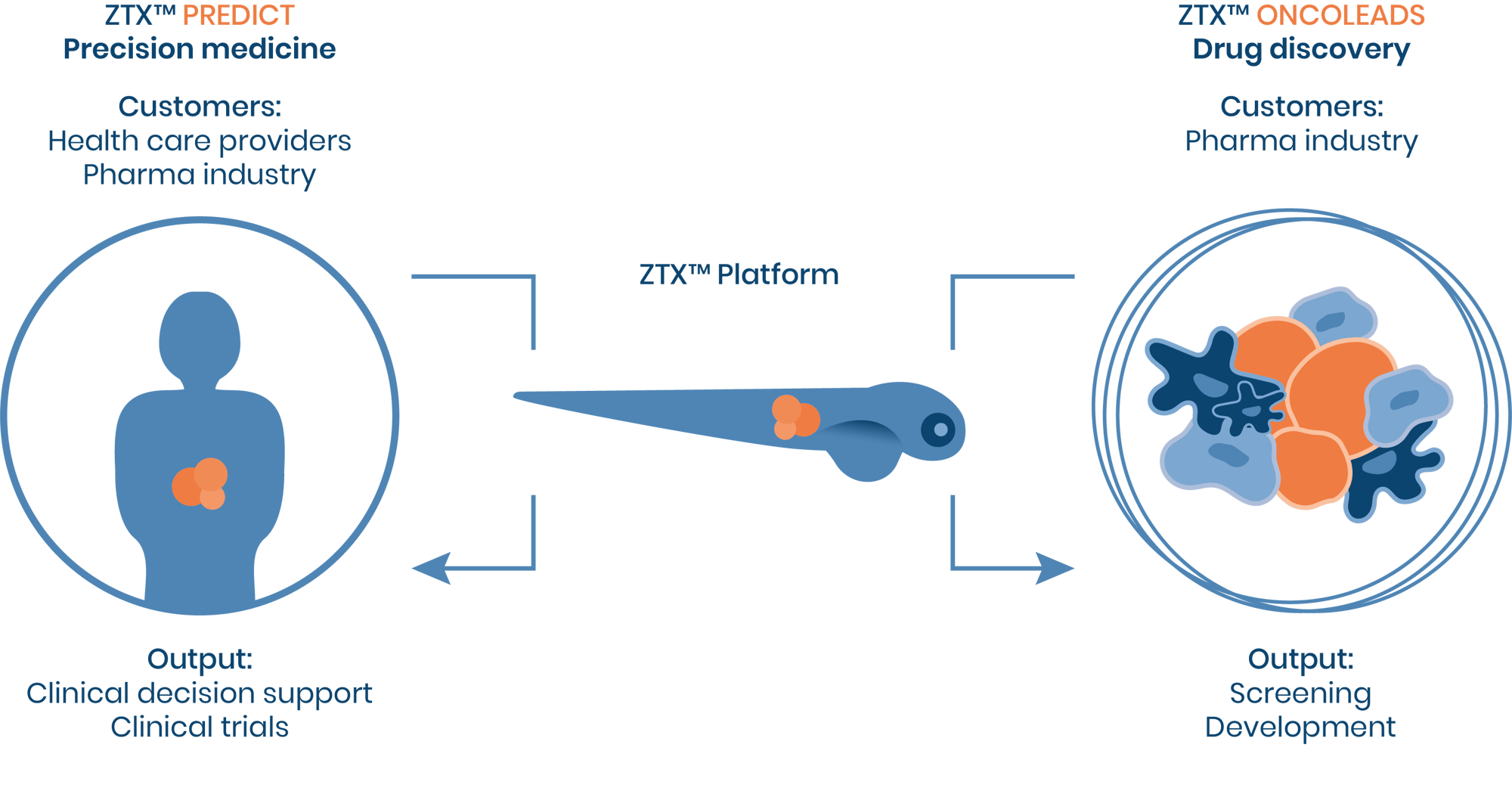
The zebrafish oncology solution
The ZTX® platform is a tool for both accelerated drug discovery and personalized medicine. The zebrafish model is used to produce oncologic xenografts which are then used in PDX, CDX, and primary patient tumors to discover and validate oncologic treatment outcomes.
Zebrafish as an oncology model
- The fast development of the zebrafish embryo enables rapid vascularization of a developed microtumor.
- The solid tumor becomes vascularized and a tumor microenvironment can be reconstituted within 3 days, both of which allow metastasis invasion.
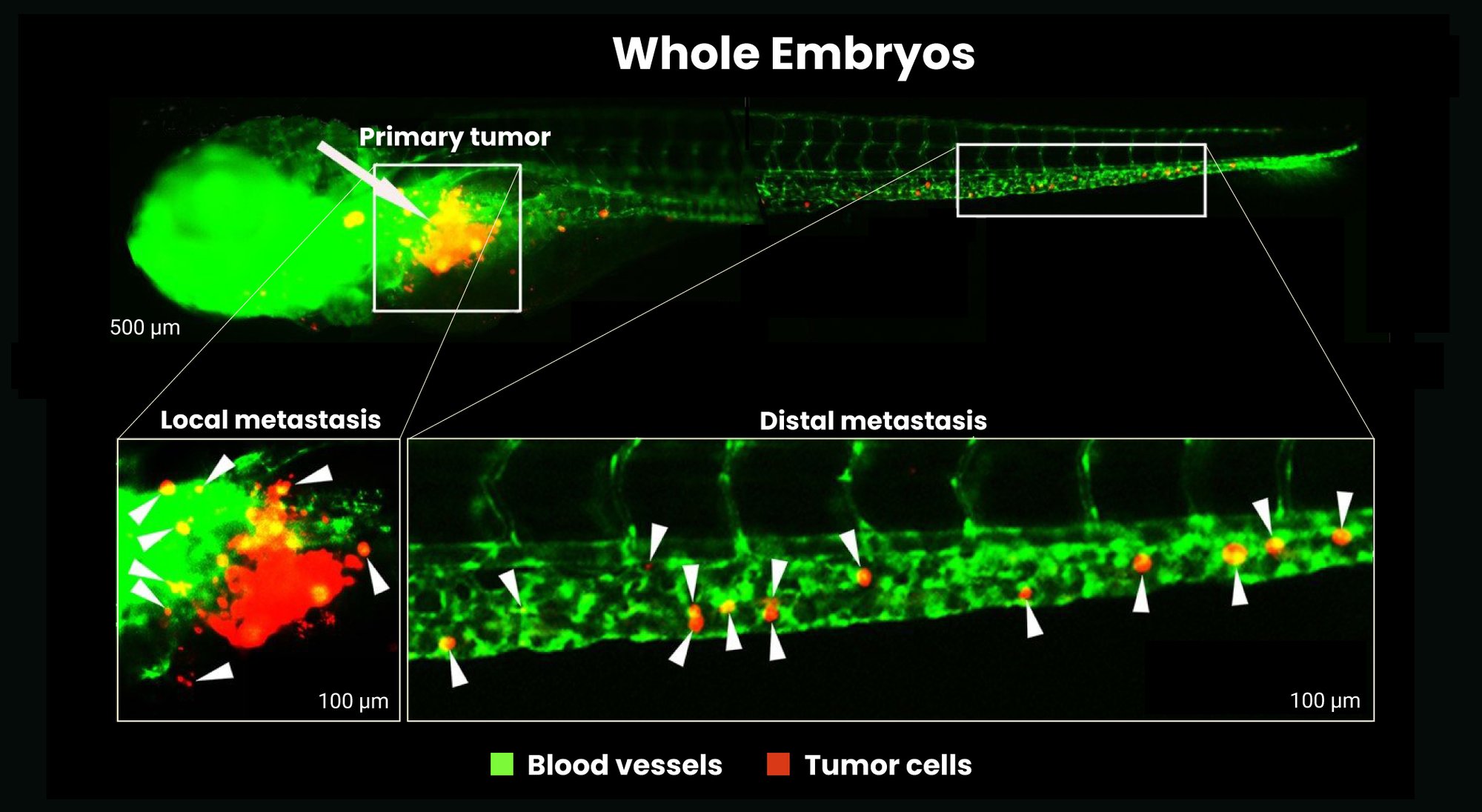
Platform Benefits
>80% specificity and sensitivity for advanced therapeutic outcomes
-
Evaluation of immuno-therapies
The ZTX® platform is being used to evaluate efficacy of T-cell and other immune cell cytotoxicity activating/boosting drugs including checkpoint inhibitors and autologous therapies within immune-oncology.
-
Human immune cell hosting
The platform effectively hosts human immune cells without any alterations, making it a powerful tool for exploring any immune-oncology research requiring validation.
-
Fast-tracked CDX and PDX modeling
The solid microtumor cells formed within the zebrafish embryo are cultured using CDX or PDX models, and even primary tumors from patients. More than 80 PDX models are validated with the ZTX® platform.
-
Applicable to all compound types
Small molecules, large peptides, antibodies, and ADCs, and nanoparticles may be tested for efficacy.
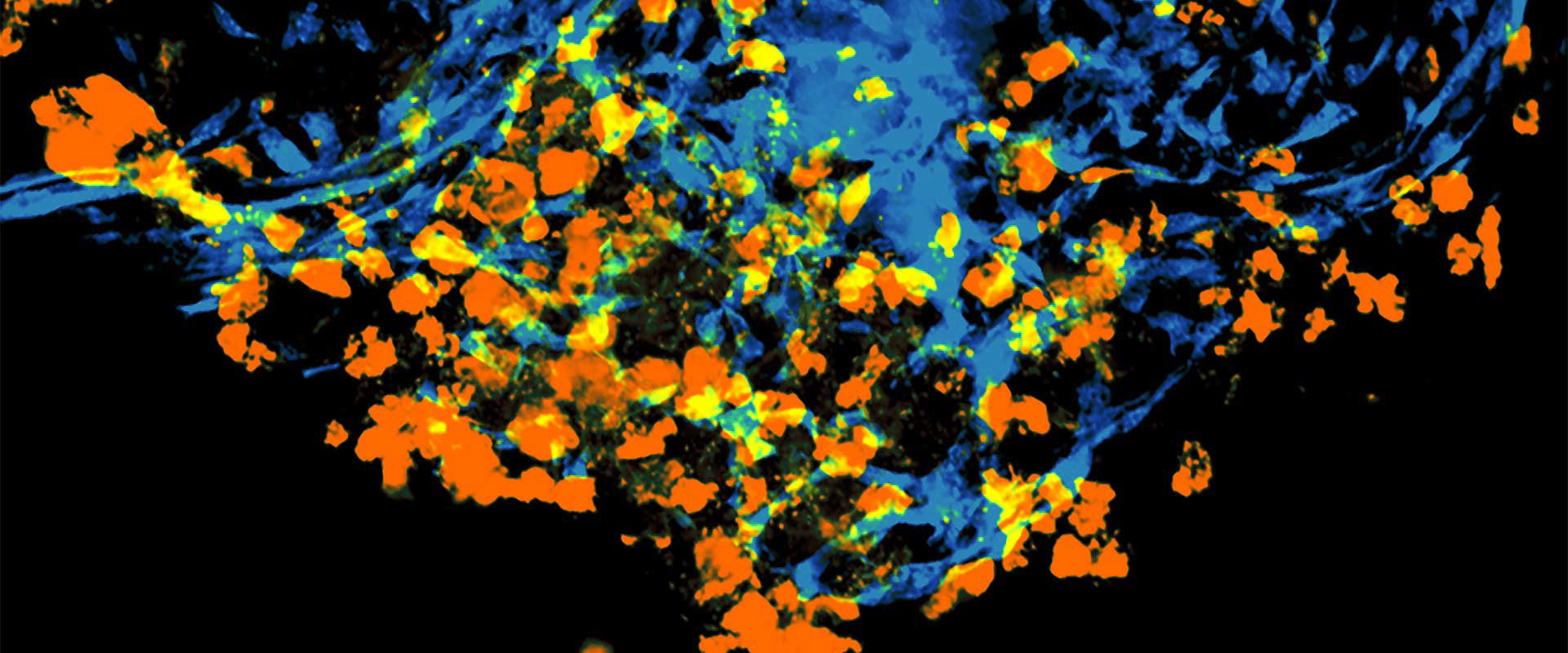
Validation of therapeutic outcome at record speed
- The ZDX® platform is a reliable companion diagnostic tool to use in CDX and PDX studies. The platform has a 79% treatment correlation to traditional PDX therapeutic validation outcome, while cutting up to 98% of the average validation study time.
Learn More about the ZTX® Platform:

